
An FDA-cleared device that reduces the physical and emotional strain of withdrawal experienced by those suffering from opioid use disorder.
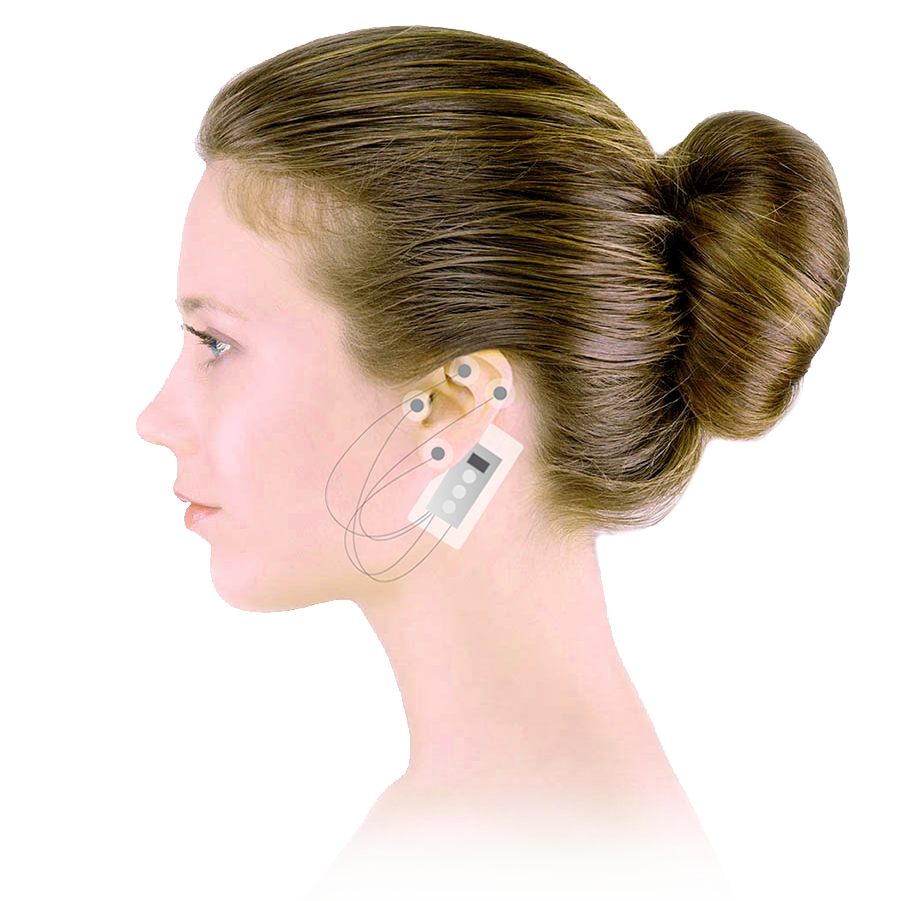
About S.T. Genesis
Our FDA-cleared Percutaneous Nerve Field Stimulator (PNFS) supports the reduction of opioid withdrawal symptoms through application to branches of cranial nerves V, VII, IX, and X as well as the occipital nerves.
This device helps patients through a noninvasive intervention directly targeting opioid withdrawal symptoms.

A Powerful Clinical Technology to Combat the Opioid Crisis
Despite extensive research regarding the addictive properties of opioid medications, the number of opioid overdose-related deaths continues to plague the country. The CDC reports that roughly 68% of the 70,200 drug overdose deaths in 2017 involved opioid use.
With millions of people receiving opioid medications as a first-line treatment for pain, the epidemic continues to harm individuals who are unable to find alternatives to alleviate their pain and opioid withdrawal symptoms. This places an immense emotional burden on those suffering due to stressful relationships with friends and family. Not only that, our society also shoulders an incredible financial burden due to this unresolved crisis. The opioid epidemic cost the U.S. economy at least $631 billion from 2015 to 2018, according to a Society of Actuaries’ (SOA) analysis of non-medical opioid use during this timeframe.
Many people seeking treatment for opioid addiction are unsuccessful due to severe withdrawal symptoms that may lead to relapse. These inherent challenges lead many to seek non-opioid drug treatments to break the cycle of addiction. This is where S.T. Genesis can help.
Stat 1: Overdose, O. (2017). Understanding the epidemic. Centers for disease control and prevention.
Stat 2: https://www.managedhealthcareexecutive.com/news/financial-burden-opioid-epidemic
Patient Scenarios S.T. Genesis Can Help
In many cases, people who are addicted to opioids have familial complications from past attempts to go through recovery programs. These patients want to stop using opiates but are fearful of relapse as their withdrawal symptoms continue to worsen. These physical symptoms associated with opioid withdrawal contribute to relapse rates, which can be detrimental to the wellbeing of the patient. S.T. Genesis percutaneous nerve field stimulation (PNFS) device may be an appropriate intervention for opioid withdrawal.
For example, a 37-year old woman presented to a local recovery center with complaints of anxiety, abdominal cramping, and vomiting. She reported a history of heroin addiction and noted that she was unsuccessful in two previous recovery programs. As part of her previous treatment, physicians prescribed her 4mg buprenorphine which was titrated up to 16 mg to reduce her opiate dependence. She reported that although this was an effective short-term solution, her cravings would return within 2-3 days, leading to relapse.
During an examination, the physician confirms she is displaying visible withdrawal symptoms (such as sweating, increased pulse rate, dilated pupils, nausea, vomiting) and measures her Clinical Opiate Withdrawal Scale (COWS) score. She scored a 38 on the COWS, suggesting severe withdrawal symptoms. The physician determined that a PNFS device could be placed for treating her severe withdrawal symptoms.
Although the PNFS device is not a cure, it serves as a bridge from successful detox to complete recovery. S.T. Genesis can support patients in this high addiction, high likelihood of relapse situation to help increase their chance of successful treatment.
Opioid use disorder sometimes stems from being prescribed opioid medications as part of a treatment regimen. Patients who are prescribed opioids to manage the pain will eventually need to increase their dosage because their tolerance builds up, eventually leading to dependence. S.T. Genesis percutaneous nerve field stimulation (PNFS) device may be an appropriate intervention for opioid withdrawal.
For example, a 58-year old man presented to a substance abuse treatment program with complaints of tremors, joint pain, and stomach cramps. He explains that was prescribed opioid medications as part of a treatment regimen for his stage two prostate cancer. Due to an increased morphine tolerance, his prescription was titrated to morphine sulfate ER 60mg twice daily. When he stops taking their morphine prescription, he will quickly feel withdrawal symptoms. The symptoms associated with opioid withdrawal contribute to prolonged use, but not necessarily abuse, of opioid medications. Once the physician examines his COWS score, she will be able to know if the S.T. Genesis can be used as an appropriate intervention for his withdrawal symptoms. In this example, the man scored 22 on the COWS, indicating moderate withdrawal symptoms that could be reduced by the PNFS device.
Differing from traditional approaches, the PNFS device delivers increased treatment compliance, as appropriate device management requires initial programming and effective placement. Accordingly, the PNFS device can decrease healthcare costs for the treatment center and improve the outcome for this patient seeking treatment.
How it Works
S.T. Genesis™ PNFS is designed to administer auricular neurostimulation treatment while offering the patient a high degree of comfort and mobility. Stimulation is performed by sending electrical pulses emitted through needles strategically positioned in the ear.
Once the device is applied, the therapy begins and continues for a maximum of 120 hours. Each patient’s treatment is determined by frequent measurement of Clinical Opiate Withdrawal Scale (COWS) feedback.
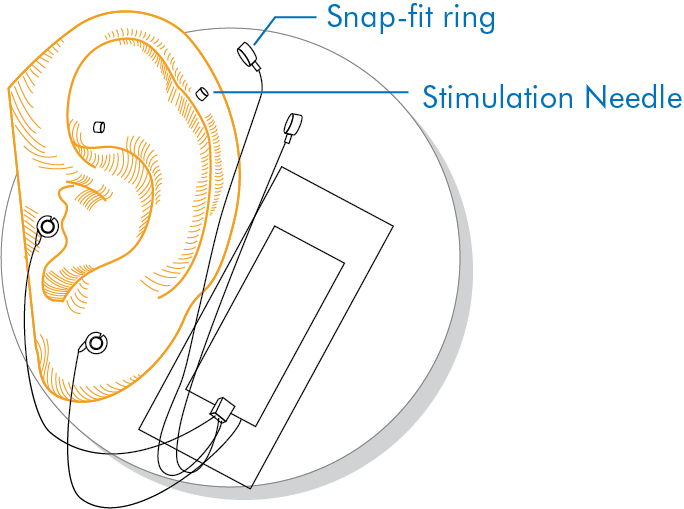
Instructions for Use of the S.T. Genesis Percutaneous Nerve Field Stimulator Device
1. Clean and disinfect the surface of the ear and skin behind the ear.
2. Project the transilluminator to locate and mark the stimulation point for cranial nerve branches V, VII, IX and X on the surface of the skin.
3. Fix the stimulation needle with the S.T. Genesis Device.
4. Activate the S.T. Genesis device.
5. Peel off the protective file from the electrode, and apply the needle to the center of the marked stimulation point.
6. Add additional adhesive over the S.T. Genesis device control module, and wire for additional strength.
Clinical Evidence
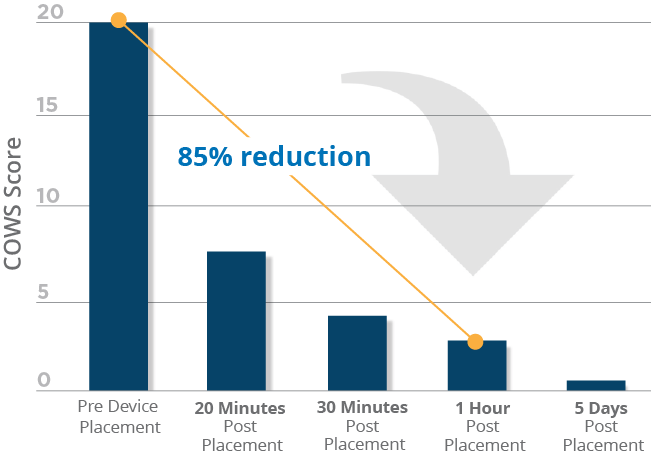 Neuromodulation with percutaneous electrical nerve field stimulation is associated with a reduction in the signs and symptoms of opioid withdrawal in a multisite, retrospective assessment.
Neuromodulation with percutaneous electrical nerve field stimulation is associated with a reduction in the signs and symptoms of opioid withdrawal in a multisite, retrospective assessment.
Percutaneous Nerve Field Stimulation is a low-risk, non-invasive therapy that has been shown to reduce opioid withdrawal symptoms and associated Clinical Opioid Withdrawal Scale (COWS) scores. Clinical evidence is available utilizing a device that functions similarly to the S.T. Genesis device to stimulate peripheral cranial neurovascular bundles in the external ear. The data shows that nearly all adult patients included in the study successfully transitioned from the use of the device during detoxification (medically supervised opioid withdrawal) to medication assisted therapy (MAT). A reduction in COWS scores was noticeable within 20 minutes of device placement, with scores continuing to drop over the five-day duration of opioid withdrawal treatment. This effective approach has been shown to allow patients to progress with recovery with the support of physician follow-up along with MAT.*
*Adrian Miranda & Arturo Taca (2018), The American Journal of Drug and Alcohol Abuse, 44:1, 56-63.
Lorem ipsum dolor sit amet
Ut wisi enim ad minim veniam, quis nostrud exerci tation ullamcorper suscipit lobortis nisl ut aliquip ex ea commodo consequat.
- Duis autem vel eum iriure dolor in
- Hendrerit in vulputate velit esse
- Consequat, vel illum dolore eu feugiat
Nulla facilisis at vero eros et accumsan et iusto odio dignissim qui blandit praesent luptatum zzril delenit augue duis dolore te feugait nulla facilisi.
30%
Lorem ipsum dolor sit amet conse tetuer adipiscin.
We Help Remove the Financial Barriers for S.T.Genesis Treatment
At Speranza we understand the difficulties in navigating the pathways to insurance reimbursement within the neuromodulation industry. That’s why we created a separate division within Speranza called Reimbursement Support Services. This division has been built with the patient and provider in mind to create seamless options to reimbursement and to ease the burden of obtaining proper reimbursement for the neuromodulation patient.
Our options include ‘do it yourself’ (information about proper codes and reference guides to help the provider better navigate proper billing for their patients), preauthorization, and specialty pharmacy services. Below are more details on each of these options.

